ABOUT US
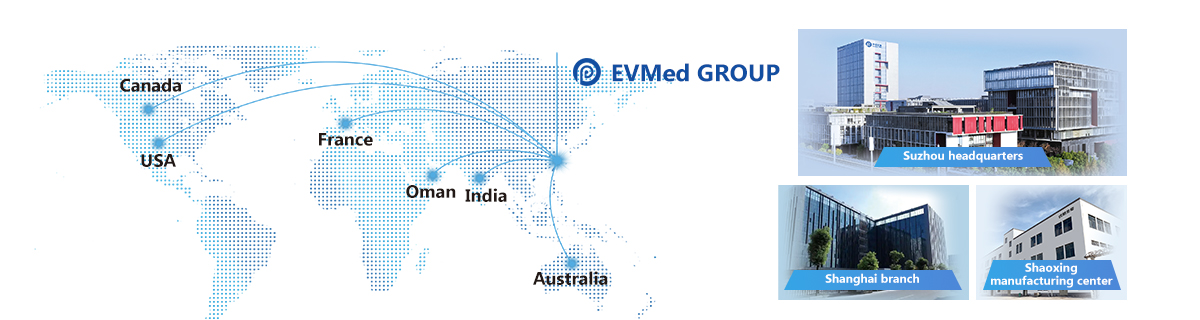
EVMed Group Biotechnology operates a state-of-the-art GMP-certified production facility spanning more then 3,000 square meters. The company has implemented a rigorous GLP and GMP-compliant quality management system to ensure the highest standards of production. With Suzhou headquarters, Shanghai Umibio branch and Shaoxing Youming manufacturing center, EVMed Group has developed a diversified business portfolio that includes pharmaceutical research and development, raw materials, finished dosage forms, and reagents, all centered around its core technology of exosome-based products.
Company History
Suzhou headquarters
Shanghai branch
Shaoxing manufacturing center